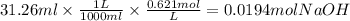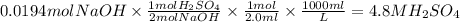Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3


Chemistry, 23.06.2019 14:00, lilyella1004
How does electronegativity changes as we move from left to right across a period
Answers: 2

Chemistry, 23.06.2019 21:30, mary9776
Draw the structures of two different compounds that have the composition ch3no2. all three h atoms must remain bonded to the c atom and both o atoms must remain bonded to the n atom.. draw the molecules by placing atoms on the grid and connecting them with bonds. include all hydrogen atoms and nonbonding electrons.
Answers: 2
You know the right answer?
The molarity of sulfuric acid in a fully charged car battery is 5.2 m. when fully discharged the mol...
Questions in other subjects:










 , where
, where 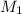 is the molarity of the acid sulfuric acid,
is the molarity of the acid sulfuric acid, 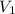 the volume of acid,
the volume of acid, 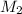 is the molarity of the base sodium hydroxide and
is the molarity of the base sodium hydroxide and 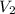 is the volume of the base.
is the volume of the base.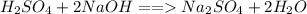 .
. 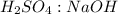 is 1:2. Which means 1 mol
is 1:2. Which means 1 mol 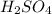 react with 2 mol
react with 2 mol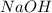 . Using the volume and molarity of
. Using the volume and molarity of