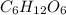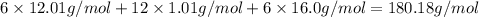Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2


You know the right answer?
Given the atomic weights of carbon, 12.01; hydrogen, 1.01; and oxygen, 16.0, what is the molar mas...
Questions in other subjects:

English, 19.01.2021 23:40




History, 19.01.2021 23:40

Mathematics, 19.01.2021 23:40

English, 19.01.2021 23:40

Mathematics, 19.01.2021 23:40

Mathematics, 19.01.2021 23:40

French, 19.01.2021 23:40