
Chemistry, 22.07.2019 00:50 bullsfan4584
Consider the chemical equilibrium of the following reaction ch3cooh ch3coo– (aq) + h+(aq) what will happen to the chemical equilibrium of the solution if ch3coona is added? the equilibrium will shifts to the right. the equilibrium will shifts to the left. the equilibrium will be unaffected. the equilibrium will be lost.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1


Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Consider the chemical equilibrium of the following reaction ch3cooh ch3coo– (aq) + h+...
Questions in other subjects:

Mathematics, 26.09.2021 14:20

Engineering, 26.09.2021 14:20


Biology, 26.09.2021 14:20

Mathematics, 26.09.2021 14:20

Computers and Technology, 26.09.2021 14:20

English, 26.09.2021 14:20

English, 26.09.2021 14:20


Physics, 26.09.2021 14:30

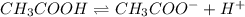
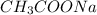 is added it will dissociate to give
is added it will dissociate to give 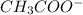 and
and 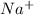
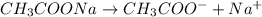
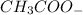 decrease i.e. the dissociation of
decrease i.e. the dissociation of 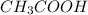 will be further depressed i.e the equilibrium will shift to the left.
will be further depressed i.e the equilibrium will shift to the left.

