
Chemistry, 20.07.2019 15:10 Benysod402
The main assumptions of the kinetic molecular theory of gases are: 1. gases are made up of molecules which are relatively far apart. 2. the molecules are in motion at high speeds. 3. the molecules are perfectly elastic. 4. increase in temperature increases the kinetic energy of the molecules. the assumption that explains charles' law is: 1 2 3 4 1 and 3

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1

Chemistry, 23.06.2019 06:30, ayoismeisjjjjuan
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1

Chemistry, 23.06.2019 08:30, lowkstahyna
What percentage of energy used in the u. s is produced from fossil fuels
Answers: 2
You know the right answer?
The main assumptions of the kinetic molecular theory of gases are: 1. gases are made up of molecule...
Questions in other subjects:

Computers and Technology, 08.01.2021 14:00



Mathematics, 08.01.2021 14:00






Mathematics, 08.01.2021 14:00

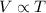 (at constant pressure)
(at constant pressure)

