Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 07:00, mahogany1956
What is the difference between covalent bonds and ionic bonds? covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between charged atoms. covalent bonds involve the transfer of electrons between charged atoms; ionic bonds involve the sharing of electrons between atoms. covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms. covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between charged atoms.
Answers: 1
You know the right answer?
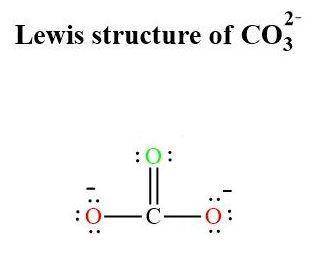
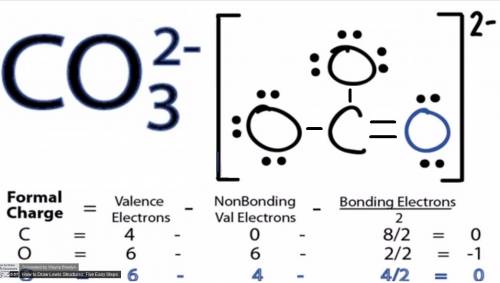
Draw the structure of co32−. include all lone pairs of electrons and formal charges....
Questions in other subjects:




Mathematics, 15.05.2021 02:10

Mathematics, 15.05.2021 02:10


Mathematics, 15.05.2021 02:10

Mathematics, 15.05.2021 02:10

Biology, 15.05.2021 02:10

Geography, 15.05.2021 02:10

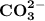 is attached in the image.
is attached in the image.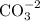 (Refer to the structure in the attached image):
(Refer to the structure in the attached image):![\begin{aligned}{\text{Total valence electrons}}\left({{\text{TVE}}} \right)&= \left[ {\left( {\text{1}} \right)\left( 4 \right) + \left( {\text{4}} \right)\left( 6 \right) + 2} \right]\\&= 30\\\end{aligned}](/tpl/images/0111/1721/6626b.png)
![{\mathbf{Formal charge=}}\left[ \begin{gathered}\left[ \begin{gathered}{\mathbf{total number of valence electrons }}\hfill\\{\mathbf{in the free atom}} \hfill \\\end{gathered} \right]{\mathbf{ }} -\\\left[ {{\mathbf{total number of non - bonding electrons}}} \right] - \\\frac{{\left[ {{\mathbf{total number of bonding electrons}}} \right]}}{{\mathbf{2}}} \\\end{gathered} \right]](/tpl/images/0111/1721/15979.png) ......(1)
......(1)![\begin{aligned}{\text{Formal charge on Carbon}} &= \left[ {4 - 0 - \frac{8}{2}} \right]\\&= 0\\\end{aligned}](/tpl/images/0111/1721/c0120.png)
![\begin{aligned}{\text{Formal charge on green O}}&=\left[ {6 - 4 - \frac{4}{2}} \right]\\&= 0\\\end{aligned}](/tpl/images/0111/1721/20d67.png)
![\begin{aligned}{\text{Formal charge on red O}}&= \left[ {6 - 6 - \frac{2}{2}} \right]\\&=- 1\\\end{aligned}](/tpl/images/0111/1721/01c54.png)