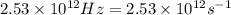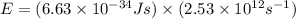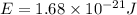Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1



Chemistry, 23.06.2019 07:20, aprilkenedy12
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
what is the energy of a photon of infrared radiation with a frequency of 2.53 × 1012 hz?...
Questions in other subjects:

History, 11.12.2020 02:20


Mathematics, 11.12.2020 02:20

English, 11.12.2020 02:20





English, 11.12.2020 02:20

Mathematics, 11.12.2020 02:20

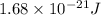
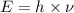
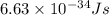
 = frequency of a photon =
= frequency of a photon =