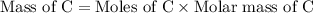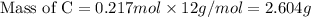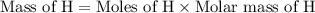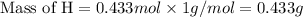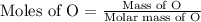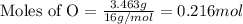Chemistry, 14.07.2019 20:50 live4dramaoy0yf9
If 6.50 g of the unknown compound contained 0.217 mol of c and 0.433 mol of h , how many moles of oxygen, o , were in the sample?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 21:30, rileydavidharless
Which substance can be broken down by chemical means
Answers: 1


Chemistry, 23.06.2019 09:30, sharmadaman641
What is the best describtion of the side of the moon that faces earth?
Answers: 2
You know the right answer?
If 6.50 g of the unknown compound contained 0.217 mol of c and 0.433 mol of h , how many moles of ox...
Questions in other subjects:



Business, 16.10.2020 09:01

English, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01


Chemistry, 16.10.2020 09:01

Mathematics, 16.10.2020 09:01