
Chemistry, 13.07.2019 13:20 wendyyy1214
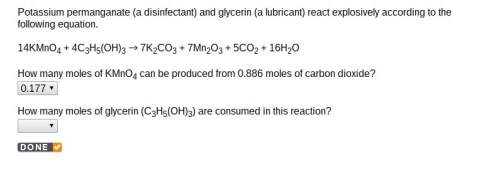
How many moles of glycerin (c3h5(oh)3) are consumed in this reaction? 14kmno4 + 4c3h5(oh)3 es001-1.jpg 7k2co3 + 7mn2o3 + 5co2 + 16h2o

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:50, iluminatioffial9699
One nanometer is equal to how many meters?
Answers: 2

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 17:10, jwhit28
Two changes are described below. a green banana turns yellow and ripens. a layer of rust forms on an iron nail. which statement is true about the two changes? a) both are chemical changes because new substances are formed. b) both are physical changes because only the physical state of the substances change. c) a is a physical change due to a change of state, but b is a chemical change because new molecules are formed. d) a is a chemical change due to a change of state, but b is a physical change because new molecules are formed.
Answers: 3
You know the right answer?
How many moles of glycerin (c3h5(oh)3) are consumed in this reaction? 14kmno4 + 4c3h5(oh)3 es001-1....
Questions in other subjects:


Mathematics, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01


History, 26.06.2020 16:01





