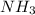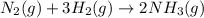Chemistry, 12.07.2019 21:00 sharonkrobinson
Write the balanced chemical equation for the haber-bosch process, that is, the combination of nitrogen and hydrogen to form ammonia, nh3. phase symbols are optional.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 21:40, fatherbamboo
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Write the balanced chemical equation for the haber-bosch process, that is, the combination of nitrog...
Questions in other subjects:

History, 03.03.2021 19:50


English, 03.03.2021 19:50

English, 03.03.2021 19:50

History, 03.03.2021 19:50

History, 03.03.2021 19:50




Biology, 03.03.2021 19:50