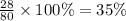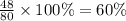The last step is to calculate the percent by mass of each element in ammonium nitrate (nh4no3). the masses of the elements in one mole of the compound are: mass n = 28.0 g mass h = 4.0 g mass o = 48.0 g the molar mass of the compound is 80.0 g/mol. what is the mass of one mole of the compound?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1


Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 19:50, nikoidurrant
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
The last step is to calculate the percent by mass of each element in ammonium nitrate (nh4no3). the...
Questions in other subjects:



Mathematics, 16.07.2019 22:20



Biology, 16.07.2019 22:20




Mathematics, 16.07.2019 22:20