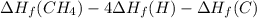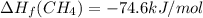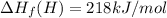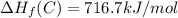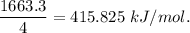Chemistry, 11.07.2019 05:50 jodonw1955
Calculate the average molar bond enthalpy of the carbon-hydrogen bond in a ch4 molecule.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

You know the right answer?
Calculate the average molar bond enthalpy of the carbon-hydrogen bond in a ch4 molecule....
Questions in other subjects:



English, 21.05.2020 22:59