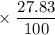Chemistry, 10.07.2019 20:50 shekinahdavis8760
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17%. 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%. show your work.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2


Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an...
Questions in other subjects:

Mathematics, 16.02.2021 19:20

English, 16.02.2021 19:20




English, 16.02.2021 19:20

Mathematics, 16.02.2021 19:20


French, 16.02.2021 19:20

Mathematics, 16.02.2021 19:20

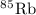 has atomic mass = 84.912 amu
has atomic mass = 84.912 amu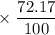
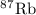 has atomic mass = 86.909 amu
has atomic mass = 86.909 amu