
Chemistry, 10.07.2019 08:30 Kayteeortiz4593
Which of the following has the largest atomic radius: f-, f, cl-, or cl?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, tatemelliott
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2


Chemistry, 23.06.2019 08:30, elijah4723
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 10:10, nancysue1975
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10-3 m and k for the dissociation is 1.86x10-5. ch3cooh(aq)+h2o(l)+> h3o+(aq)+ch3coo-(aq) show me how to get the answer.
Answers: 3
You know the right answer?
Which of the following has the largest atomic radius: f-, f, cl-, or cl?...
Questions in other subjects:


History, 22.08.2019 00:50


Chemistry, 22.08.2019 00:50

History, 22.08.2019 00:50



Mathematics, 22.08.2019 00:50

Social Studies, 22.08.2019 00:50

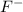 ion and there will be a total of 10 electrons in it.
ion and there will be a total of 10 electrons in it. ion. Hence, total number of electrons present in a
ion. Hence, total number of electrons present in a 

