Chemistry, 10.07.2019 02:50 lightskinbaby2
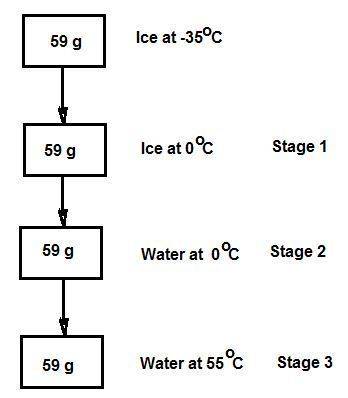
Consider the specific heats h2o(s) = 2.09 j/g · ◦c, h2o (ℓ) = 4.18 j/g · ◦c, and h2o(g) = 2.03 j/g · ◦c. the heat of fusion for water is 334 j/g and its heat of vaporization is 2260 j/g. calculate the amount of heat required to convert 59 g of ice at −35◦c completely to liquid water at 55◦c. answer in units of kj.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 23:00, emilyphillips1681
If two identical atoms are bonded, what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 05:30, Dallas3506
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

You know the right answer?
Consider the specific heats h2o(s) = 2.09 j/g · ◦c, h2o (ℓ) = 4.18 j/g · ◦c, and h2o(g) = 2.03 j/g ·...
Questions in other subjects:



Mathematics, 01.06.2021 08:20

English, 01.06.2021 08:20


Mathematics, 01.06.2021 08:20



History, 01.06.2021 08:20

Mathematics, 01.06.2021 08:20