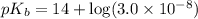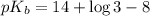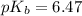Chemistry, 09.07.2019 06:30 mikaelalcool1
The k a for hypochlorous acid, hocl, is 3.0 × 10-8 at 25°c. calculate the pk b for hypochlorous anions.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, monsurviky
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 21.06.2019 20:30, sandersmakaylaovq5vu
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3


Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
The k a for hypochlorous acid, hocl, is 3.0 × 10-8 at 25°c. calculate the pk b for hypochlorous anio...
Questions in other subjects:



Law, 30.06.2020 02:01

English, 30.06.2020 02:01






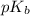 for hypochlorous anion is, 6.47
for hypochlorous anion is, 6.47 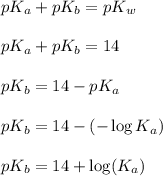
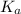 in this expression, we get the value of
in this expression, we get the value of