
Chemistry, 07.07.2019 02:20 allhailkingmilkdud
The balanced equation for combustion in an acetylene torch is shown below: 2c2h2 + 5o2 → 4co2 + 2h2o the acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2. how many moles of co2 are produced when 35.0 mol c2h2 react completely?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 11:30, nadine6085859r
Which of the following is a property of nonmetals? a. nonmetals are ductile. b. nonmetals have a shiny luster. c. nonmetals have high density. d. nonmetals are nonconductors.
Answers: 1
You know the right answer?
The balanced equation for combustion in an acetylene torch is shown below: 2c2h2 + 5o2 → 4co2 + 2h2...
Questions in other subjects:



Mathematics, 26.10.2019 20:43

Chemistry, 26.10.2019 20:43

Mathematics, 26.10.2019 20:43

Mathematics, 26.10.2019 20:43


Mathematics, 26.10.2019 20:43



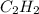 combine with 5 moles of oxygen
combine with 5 moles of oxygen 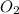 to produce 4 moles of carbon dioxide
to produce 4 moles of carbon dioxide  .
.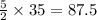 moles of oxygen
moles of oxygen 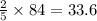 moles of acetylene
moles of acetylene 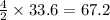 moles of of carbon dioxide
moles of of carbon dioxide 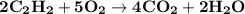
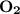 , react with
, react with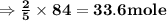 of acetone
of acetone 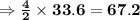 of Carbon dioxide
of Carbon dioxide 

