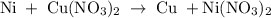Chemistry, 05.07.2019 00:00 florenciaaxell4042
Nickel metal is added to a solution of copper(ii) nitrate. express your answer as a chemical equation. identify all of the phases in your answer. enter no reaction if no reaction occurs.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1



Chemistry, 22.06.2019 05:30, MansellS5529
A3.37-mg sample of protein was chemically digested to convert its nitrogen into ammonia and then diluted to 100.0 ml. then 10.0 ml of this solution was placed in a 50-ml volumetric flask and treated with 5 ml of phenol solution plus 2 ml of sodium hypochlorite solution. the sample was diluted to 50.0 ml, and the absorbance at 625 nm was measured in a 1.00-cm cuvette and found to be 0.486. for reference, a standard solution was prepared from 10.0 mg of nh4cl (molar mass = 53.49 grams/mole) dissolved in 1.00 l of water. then 10.0 ml of this standard was placed in a 50-ml volumetric flask, treated in the same manner as the unknown, and the absorbance found to be 0.323. finally, a reagent blank was prepared using distilled water in place of unknown, it was treated in the same manner as the unknown, and the absorbance found to be 0.076. calculate the weight percent of nitrogen in the protein.
Answers: 1
You know the right answer?
Nickel metal is added to a solution of copper(ii) nitrate. express your answer as a chemical equatio...
Questions in other subjects:


Geography, 02.03.2020 17:57

English, 02.03.2020 17:57







 .
.