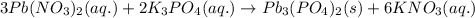Chemistry, 04.07.2019 22:50 natalie407888
When aqueous solutions of lead(ii) nitrate (pb(no3)2) and potassium phosphate (k3po4) are mixed, the products are solid lead(ii) phosphate and aqueous potassium nitrate. write the balanced chemical equation for this reaction. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, reecedstceklein
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 07:30, superfly903
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1


Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1
You know the right answer?
When aqueous solutions of lead(ii) nitrate (pb(no3)2) and potassium phosphate (k3po4) are mixed, the...
Questions in other subjects:



History, 01.08.2019 07:00

Social Studies, 01.08.2019 07:00

History, 01.08.2019 07:00