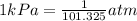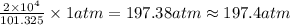Chemistry, 30.08.2019 23:30 kihgff5711
If the pressure of a gas is 2.000 × 104 kpa, what is its pressure in atm? 26.32 atm 197.4 atm 1.013 ´ 106 atm 2.026 ´ 106 atm

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

You know the right answer?
If the pressure of a gas is 2.000 × 104 kpa, what is its pressure in atm? 26.32 atm 197.4 atm 1.013...
Questions in other subjects:



Mathematics, 19.03.2020 22:23


Geography, 19.03.2020 22:23

Mathematics, 19.03.2020 22:24




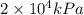 and the conversion used for the calculation is,
and the conversion used for the calculation is,