

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 06:00, jwood287375
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30, MrSavannahCat
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1
You know the right answer?
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produce...
Questions in other subjects:

Mathematics, 08.12.2020 19:10


Mathematics, 08.12.2020 19:10






Mathematics, 08.12.2020 19:10

Biology, 08.12.2020 19:10




 is a limiting reagent as it limits the formation of products and Mg is an excess reagent.
is a limiting reagent as it limits the formation of products and Mg is an excess reagent.
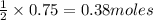 of
of 

