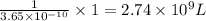Chemistry, 02.07.2019 07:00 familyvazquez7
At 25.0∘c, the molar solubility of silver chromate in water is 1.10×10−12 m . calculate the solubility in grams per liter. express your answer in grams per liter to three significant figures. how many liters of water are required to dissolve 1.00 g of silver chromate? express your answer in liters to three significant figures.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 18:00, kyllow5644
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3

You know the right answer?
At 25.0∘c, the molar solubility of silver chromate in water is 1.10×10−12 m . calculate the solubili...
Questions in other subjects:




Mathematics, 10.11.2020 23:10

History, 10.11.2020 23:10

Mathematics, 10.11.2020 23:10


History, 10.11.2020 23:10


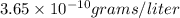 and
and 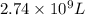 of water is required to dissolve 1 g of silver chromate.
of water is required to dissolve 1 g of silver chromate.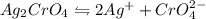
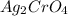 gives 2 moles of
gives 2 moles of 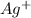 and 1 mole of
and 1 mole of 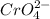
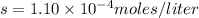
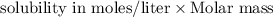
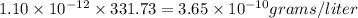
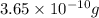 of
of