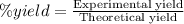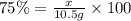Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:10, nauticatyson9
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 23:30, jade468
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
Suppose the theoretical yield in a reaction is 10.5 g and the percent yield is 75.5%. what is the ac...
Questions in other subjects:


Mathematics, 05.05.2020 05:48


English, 05.05.2020 05:48

Mathematics, 05.05.2020 05:48



Mathematics, 05.05.2020 05:48


English, 05.05.2020 05:48