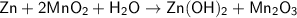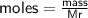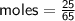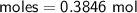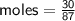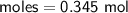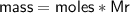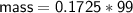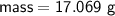Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1



Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Zn + 2MnO2 + H2O → Zn(OH)2 + Mn2O3
Determine the limiting reactant if 25g of Zn and 30g of MnO2 ar...
Questions in other subjects:

Spanish, 26.10.2020 19:40


Mathematics, 26.10.2020 19:40


Mathematics, 26.10.2020 19:40




Chemistry, 26.10.2020 19:40

Social Studies, 26.10.2020 19:40