Chemistry, 10.03.2022 06:20 mbatton879
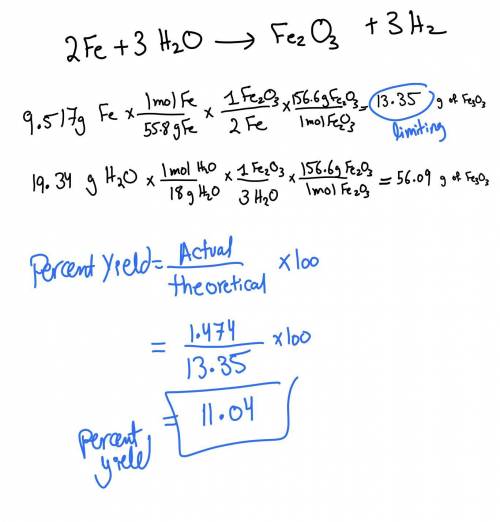
What is the limiting reagent when 9.517 g of Fe is allowed to react with 19.34 g of water according to
the reaction given below? Base your calculations on the yield of Fe2O3. How much of the excess reagent was used and left over. The actual yield of iron(III) oxide was 1.474 g. What is the percent yield?
2 Fe + 3 H20 -› Fe203 + 3 H2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, baileysosmart
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 18:00, kamjay2006
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
What is the limiting reagent when 9.517 g of Fe is allowed to react with 19.34 g of water according...
Questions in other subjects:

English, 16.11.2020 22:30


History, 16.11.2020 22:30

Physics, 16.11.2020 22:30

History, 16.11.2020 22:30

Health, 16.11.2020 22:30