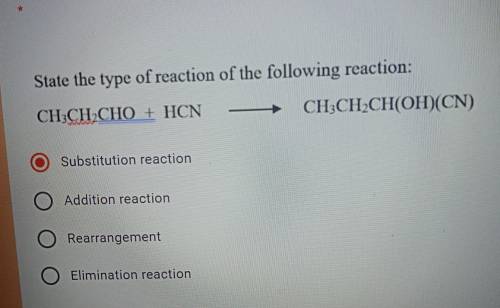
State the type of reaction of the following reaction:
A) Substitution reaction
B) Addition re...

Chemistry, 02.03.2022 23:20 matluck4162
State the type of reaction of the following reaction:
A) Substitution reaction
B) Addition reaction
C)Rearrangement
D) Elimination reaction

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 12:00, Pointjazzyqueen602
How can nonpolar molecule contain polar covalent bonds
Answers: 1

Chemistry, 23.06.2019 13:00, rachael382
How does the kinetic energy of a substance's particle in the solid phase compare to their kinetic enegy in the liquid phase?
Answers: 1
You know the right answer?
Questions in other subjects:

English, 23.02.2021 05:30


Social Studies, 23.02.2021 05:30



Mathematics, 23.02.2021 05:30

English, 23.02.2021 05:30

Mathematics, 23.02.2021 05:30

Social Studies, 23.02.2021 05:30



