
Chemistry, 26.02.2022 21:40 Sadaalcala1
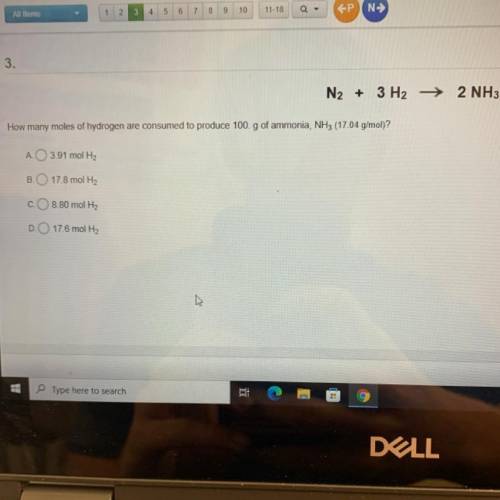
N2 + 3 H2 →2 NH3 How many moles of hydrogen are consumed to produce 100.g of ammonia, NH3 (17 04 g/mol)?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 19:10, aamu15
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 03:30, rniadsharri16
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
N2 + 3 H2 →2 NH3
How many moles of hydrogen are consumed to produce 100.g of ammonia, NH3 (17 04 g...
Questions in other subjects:



Mathematics, 01.11.2020 21:50




Physics, 01.11.2020 21:50


Mathematics, 01.11.2020 21:50

History, 01.11.2020 21:50



