
Chemistry, 23.02.2022 14:00 BreBreDoeCCx
Write the rate law for the following reaction given that the order of A = 2, B = 1, and C = 0. A + B + C ———> D + E If the concentration of C is doubled, what will happen to the rate of the reaction?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, Blaise2653
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 21:30, thompsonhomes1
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 22.06.2019 22:20, trockout4868
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
Write the rate law for the following reaction given that the order of A = 2, B = 1, and C = 0. A + B...
Questions in other subjects:

Arts, 30.08.2021 02:20




Biology, 30.08.2021 02:20

Mathematics, 30.08.2021 02:20



Mathematics, 30.08.2021 02:20

Mathematics, 30.08.2021 02:20

![\displaystyle \text{Rate} & = k[A]^2[B]](/tpl/images/2667/2602/9cfbb.png)
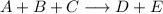
![\displaystyle \text{Rate} = k [A]^m[B]^n[C]^p](/tpl/images/2667/2602/aaf05.png)
![\displaystyle \begin{aligned} \text{Rate} & = k[A]^2[B][C]^0\\ \\ & = k[A]^2[B] \end{aligned}](/tpl/images/2667/2602/134df.png)


