
Chemistry, 22.02.2022 07:00 vapelordcarl69
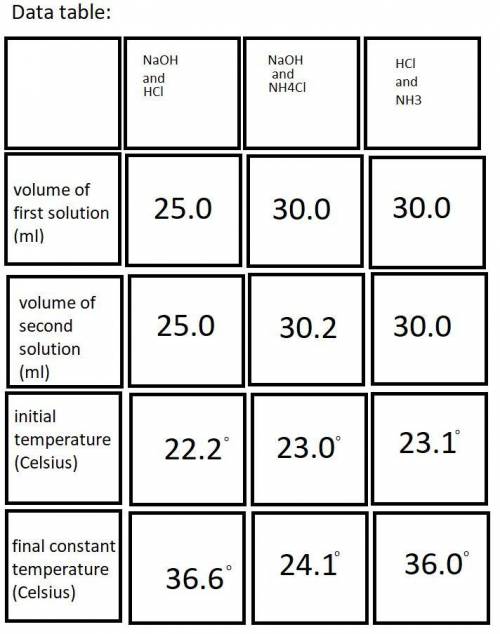
Do you have evidence from the lab that your calorimeter was not a perfect insulator? Assuming that heat was lost from the calorimeter, describe specifically how that would impact the data you recorded and then chase that change through the calculations to show how the error would impact the calculated enthalpy change for a particular reaction.
In the past, we have done this lab with roughly 50 mL each of each reactant solution, instead of 25 mL. Yet the Δ T determined experimentally were roughly the same. Explain why that is.
Each student will have slightly different volumes of reactants for each reaction. Yet the results obtained for ΔH1, ΔH2, and ΔH3 should be quite similar and would in fact be quite similar for any range of reactants combined within 25-50 mL, provided that each is accurately measured. Explain why this is the case.
A student misreads the volume of one of the solutions as 25.0 mL when in fact it was 35.0 mL. Step through the quantitative consequences of this error on the heat generated, the Δ T observed, the q calculated, and the Δ H calculated. (Clarification: Both solutions “should” be 25.0 mL or something close, and one of the volumes is accidentally 35.0 mL)
A student pours the reaction solutions into a still wet Styrofoam cup. Step through the quantitative consequences of this error on the heat generated, the Δ T observed, the q calculated, and the Δ H calculated.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3


Chemistry, 23.06.2019 06:40, donalyndearingbizz
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
Do you have evidence from the lab that your calorimeter was not a perfect insulator? Assuming that h...
Questions in other subjects:



English, 02.12.2020 19:20


Social Studies, 02.12.2020 19:20

Mathematics, 02.12.2020 19:20



English, 02.12.2020 19:20

Mathematics, 02.12.2020 19:20



