
Chemistry, 21.02.2022 07:10 nicolehathaway1012
Bismuth oxide reacts with carbon to form bismuth metal:
Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g)
When 447 g of Bi2O3 reacts with excess carbon,
(a) how many moles of Bi form?
(b) how many grams of CO form?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 14:30, KennyOaks6230
Which of the following units is not an official si unit
Answers: 1

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Bismuth oxide reacts with carbon to form bismuth metal:
Bi2O3(s) + 3C(s) → 2Bi(s) + 3CO(g)
Questions in other subjects:




Computers and Technology, 07.10.2020 23:01




Mathematics, 07.10.2020 23:01


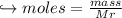
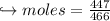
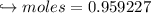
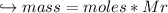
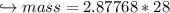
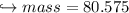 g
g

