
Chemistry, 21.02.2022 02:00 sofia467735
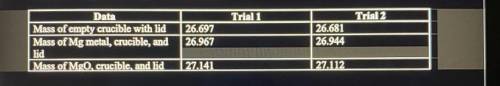
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for trial 1 and 2.
2. Determine the percent yield of MgO for trial 1 and 2.
3. Determine the average percent yield of MgO for both trials.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 02:00, Turtlelover05
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 04:20, vliu732
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for...
Questions in other subjects:

Social Studies, 10.10.2019 21:00



English, 10.10.2019 21:00



History, 10.10.2019 21:00

Social Studies, 10.10.2019 21:00

Mathematics, 10.10.2019 21:00



