
Chemistry, 14.02.2022 09:50 alexvane78
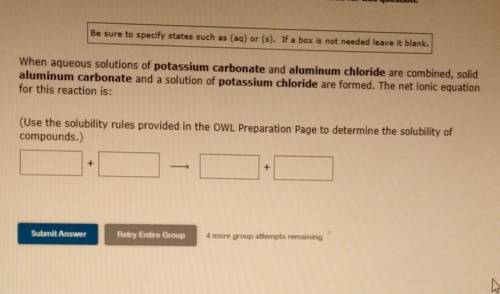
When aqueous solutions of potassium carbonate and aluminum chloride are combined, solid aluminum carbonate and a solution of potassium chloride are formed. The net ionic equation for this reaction is: (Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds.)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, ladypink94
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2



You know the right answer?
When aqueous solutions of potassium carbonate and aluminum chloride are combined, solid aluminum car...
Questions in other subjects:


Mathematics, 18.05.2021 08:10


Mathematics, 18.05.2021 08:10



Mathematics, 18.05.2021 08:10

Health, 18.05.2021 08:10


History, 18.05.2021 08:10



