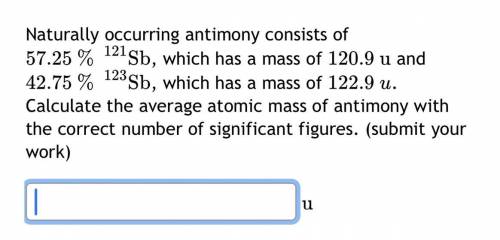
Naturally occurring antimony consists of
57.25
%
121
Sb
57.25
...

Naturally occurring antimony consists of
57.25
%
121
Sb
57.25
%
121
Sb
, which has a mass of
120.9
u
120.9
u
and
42.75
%
123
Sb
42.75
%
123
Sb
, which has a mass of
122.9
u
122.9
u
. Calculate the average atomic mass of antimony with the correct number of significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 22.06.2019 23:00, NewKidnewlessons
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3
You know the right answer?
Questions in other subjects:




Mathematics, 22.06.2019 15:40

Mathematics, 22.06.2019 15:40





English, 22.06.2019 15:40



