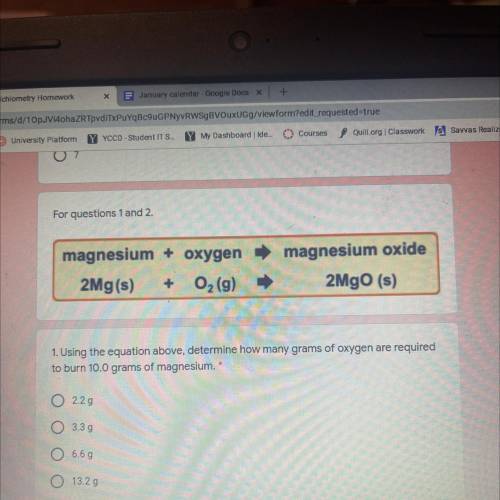
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equatio...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Cooldude3966
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3


Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3
You know the right answer?
Questions in other subjects:


History, 21.07.2019 04:23

Mathematics, 21.07.2019 04:23

Social Studies, 21.07.2019 04:23

History, 21.07.2019 04:23



History, 21.07.2019 04:23

Mathematics, 21.07.2019 04:23