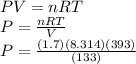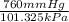Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
If I contain 1.7 moles of gas in a container with a volume of 133 liters and at a temperature of 393...
Questions in other subjects:

Mathematics, 01.12.2020 14:00

Mathematics, 01.12.2020 14:00


Mathematics, 01.12.2020 14:00


Mathematics, 01.12.2020 14:00