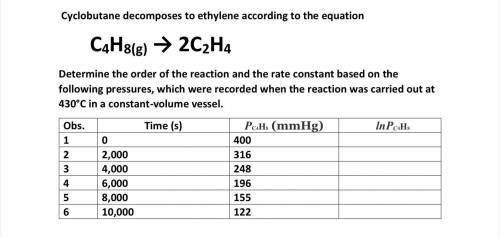
Cyclobutane decomposes to ethylene according to the equation
C4H8(g) → 2C2H4
Determine the o...

Chemistry, 24.01.2022 09:50 hurtadocrv
Cyclobutane decomposes to ethylene according to the equation
C4H8(g) → 2C2H4
Determine the order of the reaction and the rate constant based on the following pressures, which were recorded when the reaction was carried out at 430°C in a constant-volume vessel.

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 03.03.2020 01:28

Mathematics, 03.03.2020 01:28

History, 03.03.2020 01:28








