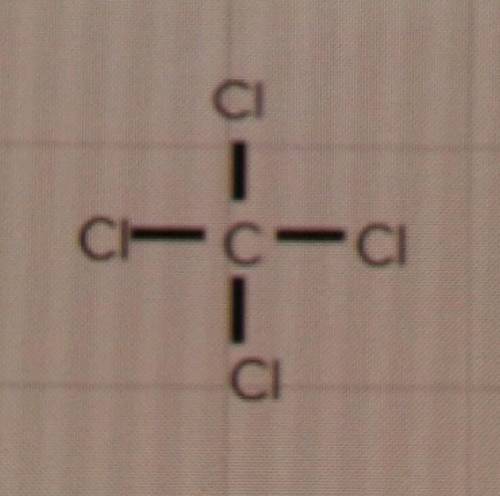Chemistry, 23.01.2022 20:50 Ilcienne6590
Hi! Can someone help me with this???
The question: Will these compounds form single, double or triple bonds?
d) CCℓ4
I'm just a bit confused because the Lewis structures look like it would be a 4 bond, but I don't know if thats even a thing. My only options are single, double or triple.
Thank you!

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 11:10, hannah2757
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:30, neidaq12345
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2
You know the right answer?
Hi! Can someone help me with this???
The question: Will these compounds form single, double or tri...
Questions in other subjects:

Mathematics, 09.11.2020 18:10

English, 09.11.2020 18:10


English, 09.11.2020 18:10


Business, 09.11.2020 18:10



Mathematics, 09.11.2020 18:10