
Chemistry, 21.01.2022 14:00 wirchakethan23
If 3.50 mol of ethane, CzH6, undergo combustion according to the unbalaced equation given of oxygen requires ? How many moles of each product formed?
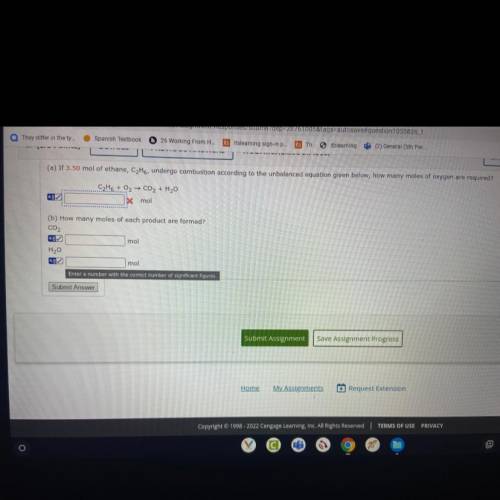

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, KarenH3512
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 09:00, Ezekielcassese
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2
You know the right answer?
If 3.50 mol of ethane, CzH6, undergo combustion according to the unbalaced equation given of oxygen...
Questions in other subjects:

Mathematics, 12.08.2021 01:50


English, 12.08.2021 01:50

Mathematics, 12.08.2021 01:50

Mathematics, 12.08.2021 02:00

Social Studies, 12.08.2021 02:00

Mathematics, 12.08.2021 02:00

Mathematics, 12.08.2021 02:00

Physics, 12.08.2021 02:00



