Question 4 (1 point)
What type of chemical reaction is the following:
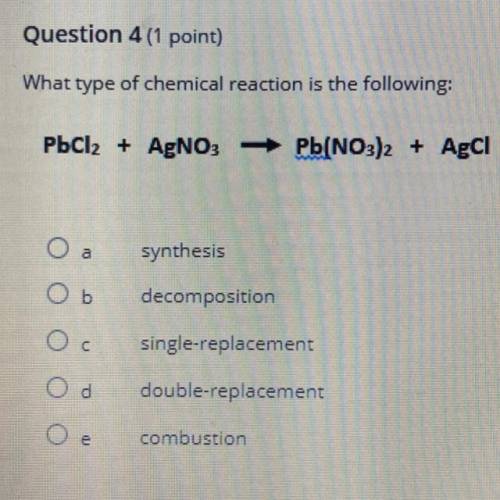
PbCl2 + AgNO3Pb(NO3)2...

Chemistry, 21.01.2022 14:00 smagallanes
Question 4 (1 point)
What type of chemical reaction is the following:
PbCl2 + AgNO3Pb(NO3)2 + Agci
a
synthesis
b
decomposition
Ос
single-replacement
Od
double-replacement
o e
combustion

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:50, Pookiev
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1


You know the right answer?
Questions in other subjects:

Mathematics, 23.08.2019 21:30

Biology, 23.08.2019 21:30


History, 23.08.2019 21:30



Mathematics, 23.08.2019 21:30

Mathematics, 23.08.2019 21:30

English, 23.08.2019 21:30

History, 23.08.2019 21:30



