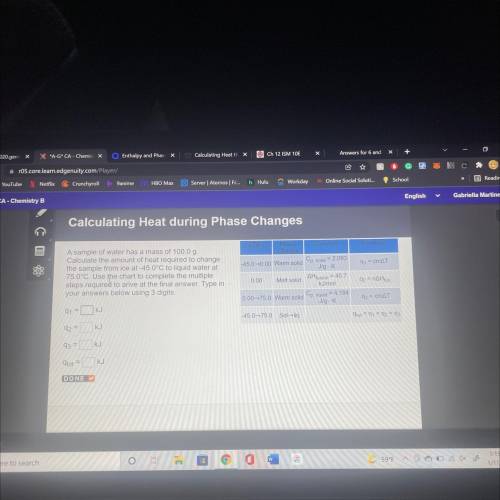
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the s...

Chemistry, 19.01.2022 14:00 somethingar183
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the sample from ice at 450°C to liquid water at
78.0°C. Use the chart to complete the multiple
steps required to arrive at the final answer. Type in your answers below using 3 digits.
Q1= KJ
Q2= KJ
Q3= KJ
q(tot)= KJ

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, miller5452
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1

Chemistry, 22.06.2019 23:30, billybob8514
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 25.02.2020 04:00







Mathematics, 25.02.2020 04:00




