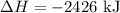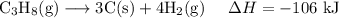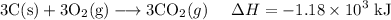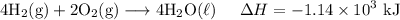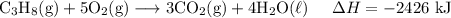H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ

Chemistry, 18.01.2022 08:50 monkeyrose1999
Study the reactions.
C (s)+O2 (g)→CO2 (g) ΔH=−394 kJ
H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ
3C (s)+4H2 (g)→C3H8 (g) ΔH=106 kJ
Target Reaction:
C3H8 (g)+5O2 (g)→3CO2 (g)+4H2O (l)ΔH= ?
What is the enthalpy change of the target reaction?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 08:30, omoaye
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3
You know the right answer?
Study the reactions.
C (s)+O2 (g)→CO2 (g) ΔH=−394 kJ
H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ
H2 (g)+12O2 (g)→H2O (l) ΔH=−286 kJ
Questions in other subjects:


English, 27.09.2020 17:01

English, 27.09.2020 17:01



Mathematics, 27.09.2020 17:01




Mathematics, 27.09.2020 17:01