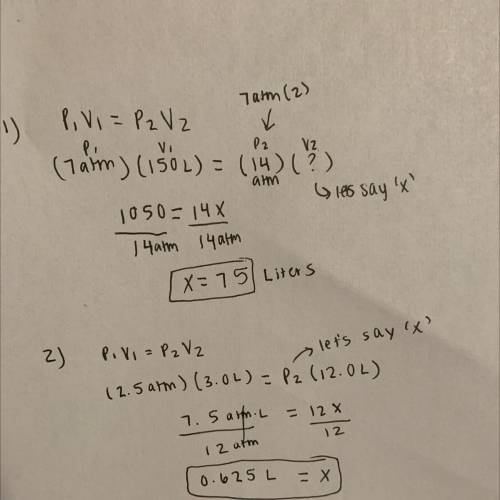Chemistry, 16.01.2022 07:50 sullivanjakob
1.A gas sample has a volume of 150. L when the pressure is 7.00 atm. If the temperature and amount of gas remains constant, what volume will the gas sample occupy at a pressure is doubled?
2. A helium gas in a balloon occupies 3.0 L at 2.5 atm. At what pressure will it point occupy 12 L assuming the mass and the temperature are constant?
Please answer correctly with evidence both parts

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
1.A gas sample has a volume of 150. L when the pressure is 7.00 atm. If the temperature and amount o...
Questions in other subjects:

Mathematics, 28.01.2020 16:40


Mathematics, 28.01.2020 16:40




Biology, 28.01.2020 16:40