Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
...

Chemistry, 12.01.2022 01:00 Dreambig85
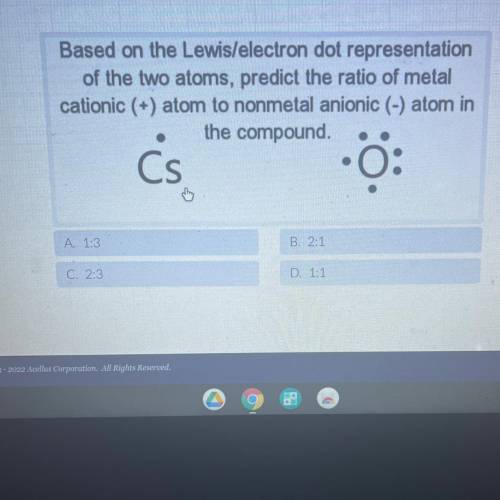
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
cationic (+) atom to nonmetal anionic (-) atom in
the compound.
Cs
0:
A. 1:3
B. 2:1
C. 2:3
D. 1:1

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, valencial0917
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 04:00, ayoismeisjjjjuan
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Questions in other subjects:












