
Chemistry, 03.01.2022 14:00 Lindseycline123
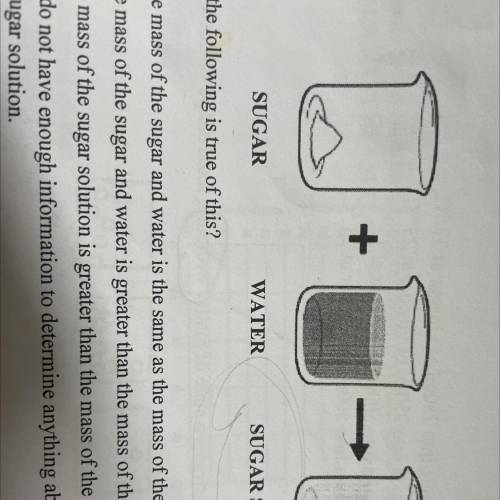
The diagram below represents the creation of a sugar solution by adding sugar to water then
stirring the mixture.
SUGAR
WATER
SUGAR SOLUTION
Which of the following is true of this?
A. The mass of the sugar and water is the same as the mass of the sugar solution.
B. The mass of the sugar and water is greater than the mass of the sugar solution.
C. The mass of the sugar solution is greater than the mass of the sugar and water.
D. We do not have enough information to determine anything about the mass of the sugar, water, or
the sugar solution.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, webbhlharryteach
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 23:00, lulprettyb
What is the most common reason for matter changing its state?
Answers: 1
You know the right answer?
The diagram below represents the creation of a sugar solution by adding sugar to water then
stirri...
Questions in other subjects:


Mathematics, 23.06.2019 22:00

History, 23.06.2019 22:00

Mathematics, 23.06.2019 22:00

Mathematics, 23.06.2019 22:00


Mathematics, 23.06.2019 22:00





