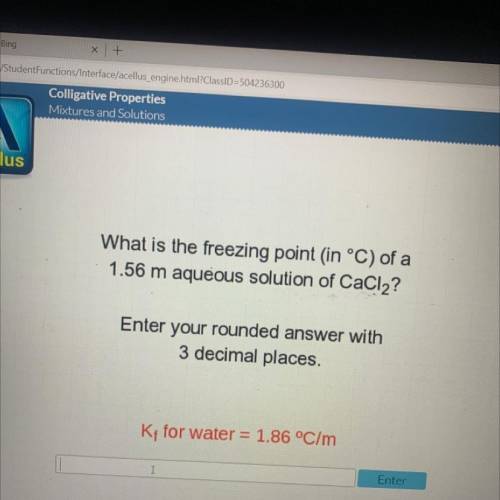
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded...

Chemistry, 02.01.2022 14:00 edimilperdomo
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
Kt for water = 1.86 °C/m

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


Chemistry, 22.06.2019 22:30, lizzzzi7908
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
Questions in other subjects:


Chemistry, 27.09.2019 13:10

History, 27.09.2019 13:10


Mathematics, 27.09.2019 13:10


Advanced Placement (AP), 27.09.2019 13:10



English, 27.09.2019 13:10



