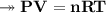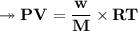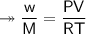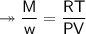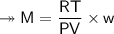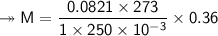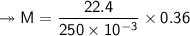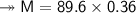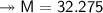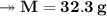At STP,250cm³of gas had a mass of 0.36g.
1. What result does this give for the molar mass of ga...

Chemistry, 01.01.2022 22:40 jojoblue2004
At STP,250cm³of gas had a mass of 0.36g.
1. What result does this give for the molar mass of gas

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:00, kamkam5791
Is powdered sports drink ionic or covalent ? 10pts !
Answers: 1


Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 23.06.2019 11:40, brendonvernon8
Which of the following would have the lowest average kinetic energy
Answers: 1
You know the right answer?
Questions in other subjects:






Social Studies, 24.12.2019 00:31


History, 24.12.2019 00:31


Business, 24.12.2019 00:31

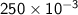 L P = 1 atmT = 273 KR = 0.0821 atm L/ mol K
L P = 1 atmT = 273 KR = 0.0821 atm L/ mol K