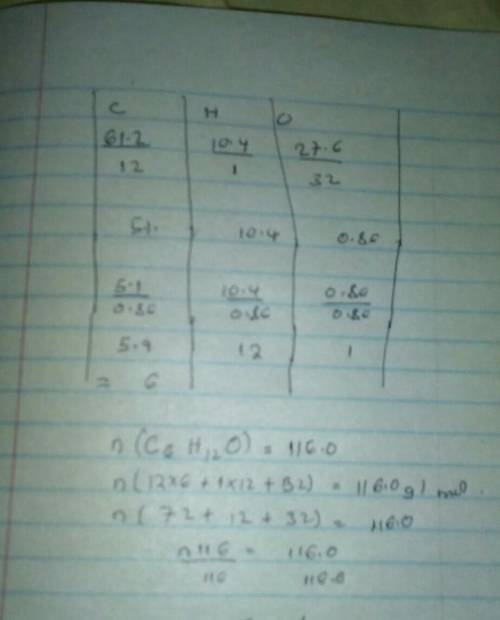Chemistry, 01.01.2022 19:50 geminigirl077
#4: Determine the molecular formula of o compound that is made up of 61.2 g carbon, 10.4 g hydrogen and 27.6 g oxygen if its molar mass is 116.0 g/mole. I will give Brainliest to best answer + SHOWS WORK!! fake answers will be reported and deleted. <3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, 20jessicacabriales
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 07:20, rex1578
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
#4: Determine the molecular formula of o compound that is made up of 61.2 g carbon, 10.4 g hydrogen...
Questions in other subjects:

Chemistry, 24.01.2022 23:50


Mathematics, 24.01.2022 23:50


Social Studies, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50

Mathematics, 24.01.2022 23:50