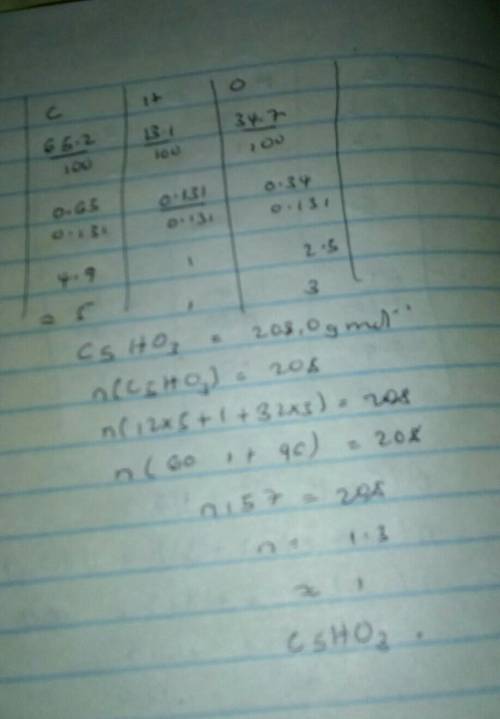Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

You know the right answer?
#6: A compound contains 65.2% carbon, 13.1% hydrogen and 34.7% oxygen. If its molar mass is 208.0 g/...
Questions in other subjects:


Physics, 08.11.2019 19:31


History, 08.11.2019 19:31



Mathematics, 08.11.2019 19:31