
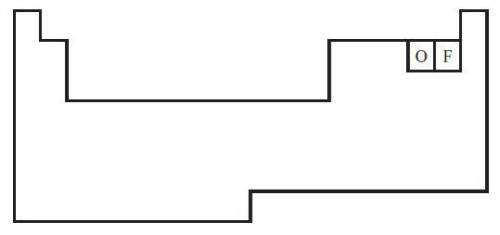
The diagram below shows a partial periodic table.
The electron configuration of oxygen is 1s2 2s2 2p4. On the periodic table, fluorine is one space to the right of oxygen. Which of the following electron configurations represents fluorine?
1s2 2s2 2p3
1s2 2s2 2p6 1s2 3s2 3p3
1s2 2s2 2p5

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 16:50, TheOriginal2x
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
The diagram below shows a partial periodic table.
The electron configuration of oxygen is 1s2 2s2...
Questions in other subjects:

Physics, 25.08.2019 06:30


History, 25.08.2019 06:30

Chemistry, 25.08.2019 06:30


Mathematics, 25.08.2019 06:30



History, 25.08.2019 06:30




