
Chemistry, 16.12.2021 01:10 blondielocks2002
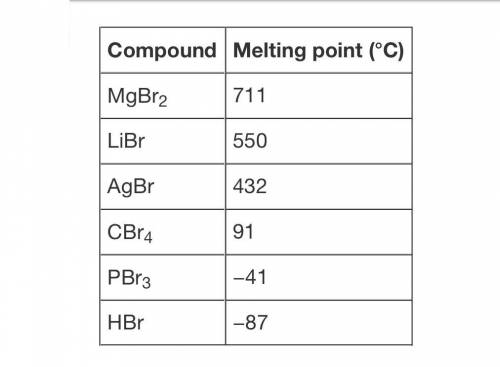
The table shows the melting points of different compounds containing the element bromine.
Which of the following statements are supported by the data? Select all that apply.
A. All of the compounds exist in the solid state at 25°C.
B. The compounds in which bromine bonds with a metal have higher melting points than the compounds in which bromine bonds with a nonmetal.
C. The compound with the highest melting point is made up of ions held together by ionic bonds.
D. The compound with the lowest melting point is made up of molecules held together by intermolecular attractions.
E. The melting point of CBr4 suggests that it can be classified as an ionic compound.
F. It takes more energy to overcome the attractive forces between particles of solid LiBr than it does to overcome the attractive forces between particles of solid PBr3.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:30, Elliendc7939
List and describe the neurological effects of the vocs and other air pollutants, as described by dr. theo colborn
Answers: 2

Chemistry, 23.06.2019 05:50, starfox5454
Which of the following isotopes has the same number of neutrons as phosphorus-31?
Answers: 1
You know the right answer?
The table shows the melting points of different compounds containing the element bromine.
Which of...
Questions in other subjects:


Mathematics, 13.09.2021 14:00



Mathematics, 13.09.2021 14:00

Mathematics, 13.09.2021 14:00


Advanced Placement (AP), 13.09.2021 14:00

Chemistry, 13.09.2021 14:00




