
Chemistry, 15.12.2021 02:30 Lenaaa2019
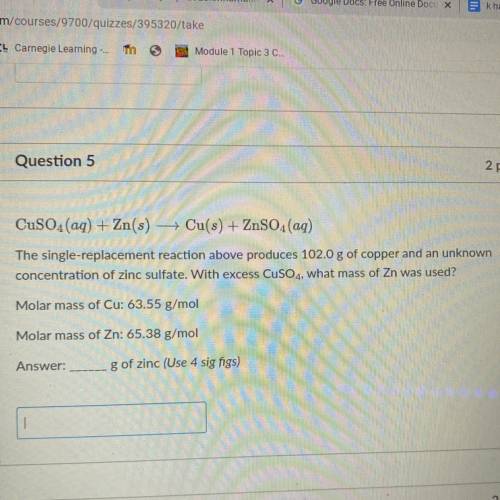
CuSO4(aq) + Zn(s) — Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 102.0 g of copper and an unknown
concentration of zinc sulfate. With excess CuSO4, what mass of Zn was used?
Molar mass of Cu: 63.55 g/mol
Molar mass of Zn: 65.38 g/mol
g of zinc (Use 4 sig figs)

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 06:30, tdahna0403
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
CuSO4(aq) + Zn(s) — Cu(s) + ZnSO4(aq)
The single-replacement reaction above produces 102.0 g of co...
Questions in other subjects:

Social Studies, 04.07.2019 05:40





Mathematics, 04.07.2019 05:40

Chemistry, 04.07.2019 05:40



Mathematics, 04.07.2019 05:40



